Related Questions
Q33: What would a pressure reading of 565
Q34: A sample of a gas in a
Q35: A gas-filled balloon on a warm day
Q36: Calculate the density of CO gas at
Q37: A mixture of 5.00 moles of neon
Q39: At 298 K a sample of 2.00
Q40: A gas sample weighing 3.78 grams occupies
Q41: A sample of an unknown gas was
Q42: A gas sample containing 0.3250 moles of
Q43: Dry air contains 78% N<sub>2</sub>, 21% O<sub>2</sub>,
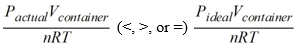 for a real gas.
for a real gas.