Short Answer
In a gas mixture containing oxygen, neon, and argon, the mole fractions are: 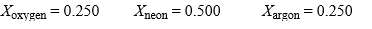 If this mixture behaves as an ideal gas, what is the density of this mixture, in g/liter, at 37.0 °C and 765 torr? If someone gave a sample of the mix described above to a student and had them determine the molar mass of the "gas"without telling the student it was a mixture, what value should the student report?
If this mixture behaves as an ideal gas, what is the density of this mixture, in g/liter, at 37.0 °C and 765 torr? If someone gave a sample of the mix described above to a student and had them determine the molar mass of the "gas"without telling the student it was a mixture, what value should the student report?
Correct Answer:

Verified
1.11 g L−1, ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q11: A sample of hydrogen gas is placed
Q12: People who live on the second floor
Q13: The van der Waals equation of state
Q14: A gas sample containing 0.3525 moles of
Q15: A gas container has a volume of
Q17: A sealed glass container contains 0.8 moles
Q18: A properly designed Torricelli mercury barometer should
Q19: A U.S. Weather Bureau forecast cited the
Q20: Five moles of oxygen gas are heated
Q21: How many liters of oxygen gas, measured