Multiple Choice
Consider the cyclobutadiene structure shown below. How many bonds are there? 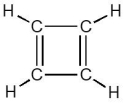
A) 1
B) 2
C) 3
D) 4
E) 8
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q168: What is the shape of the boron
Q169: What is the shape of the PH<sub>3</sub>
Q170: Which of the following molecules or ions
Q171: What type of hybrid orbitals are
Q172: A molecule has a measurable dipole moment.
Q174: Which of the following statements is true?<br>A)Magnesium
Q175: The <span class="ql-formula" data-value="\pi"><span class="katex"><span
Q176: Select the species which has the largest
Q177: The two vertical bonds in a trigonal
Q178: Kevin, the high school whiz kid, is