Multiple Choice
Consider a hypothetical species with an octahedral geometry shown below, with three different placements of its bonded atoms (indicated as "A") and lone pairs (indicated as blank) . 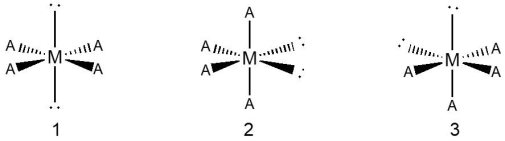 Which species, 1, 2, or 3, is the preferred orientation of atoms and lone pairs?
Which species, 1, 2, or 3, is the preferred orientation of atoms and lone pairs?
A) 1
B) 2
C) 3
D) 2 and 3 are equally probable.
E) None of these arrangements of atoms and lone pairs is possible.
Correct Answer:

Verified
Correct Answer:
Verified
Q84: Predict the molecular geometry and polarity of
Q85: In molecular orbital theory, the extra stability
Q86: The I<sub>3</sub><sup>-</sup> ion has a total of
Q87: Compare the molecules SbCl<sub>5</sub> and IF<sub>5</sub>, using
Q88: Which of the five basic geometries for
Q90: The metaphosphate ion, PO<sub>3</sub><sup>-</sup>, has a total
Q91: The energy separation between the nearest empty
Q92: What is the shape of the SF<sub>4</sub>
Q93: The C<sub>2</sub>H<sub>4</sub> molecule has hybrid orbitals centered
Q94: According to molecular orbital theory, the bond