Multiple Choice
A student drew four possible Lewis structures for HBrO4 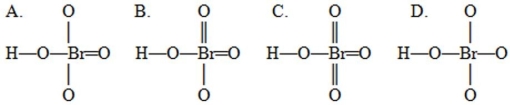 Complete these Lewis structures presented above by filling in the remaining valence electrons that are not in the bonds. Based on these structures, the preferred structure would be the structure shown as ________ in which the sum of the absolute values of the formal charges on all the atoms is ________. Hint: When determining remaining valence electrons remember to account for the electrons already present in the bonds.
Complete these Lewis structures presented above by filling in the remaining valence electrons that are not in the bonds. Based on these structures, the preferred structure would be the structure shown as ________ in which the sum of the absolute values of the formal charges on all the atoms is ________. Hint: When determining remaining valence electrons remember to account for the electrons already present in the bonds.
A) A, 4
B) B, 2
C) C, 0
D) D, 6
E) D, 0
Correct Answer:

Verified
Correct Answer:
Verified
Q6: There are three resonance structures for the
Q7: The chlorite ion has a Lewis structure
Q8: Draw the Lewis structure for SOCl<sub>2</sub>. Considering
Q9: Sodium forms a monatomic ion that has
Q10: Which bond is the most polar?Hint: Consider
Q12: The Lewis symbol for the carbon atom
Q13: Draw the most favorable Lewis structure for
Q14: The formal charge on the most stable
Q15: Which of the following solids would have
Q16: Which of these species, Fe<sup>3+</sup>, P<sup>3−</sup>, B<sup>−</sup>,