Multiple Choice
Calculate the energy required to excite a hydrogen atom by causing an electronic transition from the energy level with n = 1 to the level with n = 4. Recall that the quantized energies of the levels in the hydrogen atom are given by: 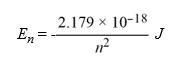
A) 2.02 × 10-29 J
B) 2.04 × 10-18 J
C) 2.19 × 105 J
D) 2.25 × 10-18 J
E) 3.27 × 10-17 J
Correct Answer:

Verified
Correct Answer:
Verified
Q73: When one thinks of the size of
Q74: Which placement of electrons is never encountered
Q75: How many completely filled subshells are there
Q76: What is the frequency of radiation which
Q77: How many orbitals are in the shell
Q79: The ground-state of _ has an electron
Q80: Which statement correctly summarizes allowed values of
Q81: An electron in an atom can be
Q82: Which atom has the most exothermic electron
Q83: The Pauli principle states that<br>A)an electron in