Multiple Choice
Calculate the wavelength, in nanometers, of light emitted by a hydrogen atom when the electron falls from an n = 7 energy level to an n = 4 energy level. Recall that the quantized energies of the levels in the hydrogen atom are given by: 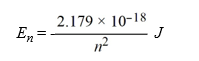
A) 4.45 × 10-20 nm
B) 8.51 × 102 nm
C) 2.17 × 103 nm
D) 1.38 × 1014 nm
E) 2.16 × 103 nm
Correct Answer:

Verified
Correct Answer:
Verified
Q144: The de Broglie relationship provides a link
Q145: The following are all ionization energies for
Q146: What is the energy of one photon
Q147: Which statement best describes the d orbitals?<br>A)There
Q148: What is the energy of one mole
Q150: A possible set of quantum numbers for
Q151: The diffraction of electrons is the principle
Q152: There are several possible arrangements of electrons
Q153: Kevin is working on a summer project
Q154: A carbon atom has _ valence electrons.