Short Answer
Given the following sets of quantum numbers for n, l, ml, and ms, which one of these sets is not possible for an electron in an atom? 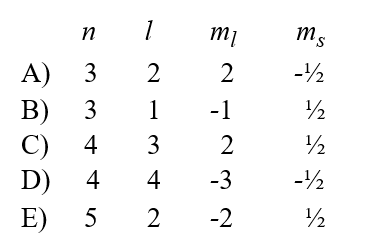
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q200: According to the photoelectric effect, increasing the
Q201: Given the following sets of quantum numbers
Q202: There are more unpaired electrons in the
Q203: Which atom in the set [O, F,
Q204: What is the maximum number of electrons
Q206: Calculate the wavelength of the spectral line
Q207: A good rule of thumb is: the
Q208: Which statement is true concerning Bohr's model
Q209: Calculate the wavelength of an electron (mass
Q210: How many electrons can be placed in