Short Answer
Given the following sets of quantum numbers for n, l, ml, and ms, which one of these sets is not possible for an electron in an atom? 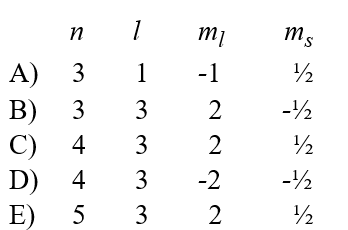
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q209: Calculate the wavelength of an electron (mass
Q210: How many electrons can be placed in
Q211: The amplitude of a wave at any
Q212: The electron microscope makes use of the
Q213: A correct description for the electron configuration
Q214: The probability of finding the electron varies
Q215: Which statement is true?<br>A)The line spectra of
Q216: Which placement of electrons is never encountered
Q217: Which of the following choices is the
Q219: In the orbital diagram for boron, the