Short Answer
A possible set of quantum numbers for an electron in the partially filled subshell in a germanium atom in its ground state configuration would be 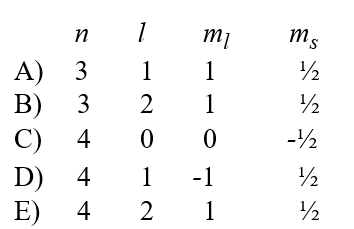
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q54: A nitrogen atom has how many valence
Q55: Which radiation has the shortest wavelength?<br>A)gamma rays<br>B)infrared
Q56: Which atom has the smallest radius?<br>A)Ar<br>B)Ca<br>C)K<br>D)Mg<br>E)Na
Q57: Which radiation has the highest frequency?<br>A)blue visible
Q58: For a given value of n, the
Q60: What is the energy, in joules, of
Q61: The number of electrons required to fill
Q62: What is the energy of one mole
Q63: Atomic hydrogen has a single electron, and
Q64: What is the maximum number of electrons