Short Answer
A possible set of quantum numbers for an electron in the partially filled subshell in a technetium atom in its ground state configuration would be 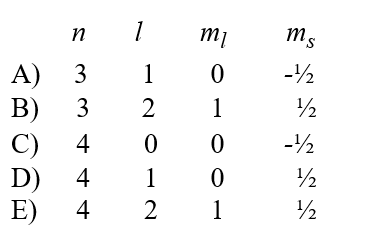
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: When n = 2, there are two
Q4: Which reaction is most exothermic?<br>A)Cl(g)+ e<sup>-</sup>
Q5: A wave in which the crests and
Q6: Which configuration represents an excited state configuration
Q7: How many unpaired electrons are in gold?<br>A)2<br>B)4<br>C)6<br>D)8<br>E)1
Q9: What is the wavelength of electromagnetic radiation
Q10: Calculate the wavelength, in meters, of a
Q11: Which atom in the set [Mg, Cr,
Q12: What is the energy of one photon
Q13: Which atom in the set [O, F,