Short Answer
What is the energy required to excite a hydrogen atom by causing an electronic transition from the n = 2 to the n = 5 energy level? Recall that the quantized energies of the levels in the hydrogen atom are given by: 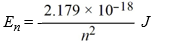
Correct Answer:

Verified
Correct Answer:
Verified
Q103: The decrease in atomic radius as one
Q104: Which atom in the set [Ba, Cr,
Q105: The electron microscope makes use of the
Q106: Which atom has the largest radius?<br>A)Rb<br>B)Na<br>C)Al<br>D)Ne<br>E)O
Q107: Which atom in the set [Y, Cr,
Q109: Which radiation has the lowest frequency?<br>A)gamma rays<br>B)infrared
Q110: A particular energy level in an atom
Q111: Which radiation has the longest wavelength?<br>A)gamma rays<br>B)infrared
Q112: The letter designation for the subshell in
Q113: Which of the following are expected to