Short Answer
The maximum number of electrons in an atom that can have the following exact same set of quantum numbers is ________. 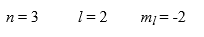
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: Which atom in the set [O, F,
Q14: The fact that the energy of paired
Q15: The set of 3d orbitals is populated
Q16: For which process is the largest
Q17: The spinning electrical charge of the electron
Q19: The spin of the electron is responsible
Q20: In an electromagnetic wave, an oscillating charge
Q21: A correct description for the electron configuration
Q22: Which atom has the largest first ionization
Q23: The frequency of a wave is related