Short Answer
A partial activity series is: Au < Pt < Ag < Cu < H2 < Sn < Co < Fe < Mg. Based on this series, which of the following solutions would cause a reaction with Cu(s)? 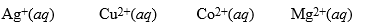
Correct Answer:

Verified
Correct Answer:
Verified
Q25: Which of the following involves reduction?<br>A)Ba<sup>2+</sup>(aq)+
Q26: When H<sub>2</sub>O<sub>2</sub> behaves as a reducing agent,
Q27: The reaction, AgNO<sub>3</sub>(aq)+ NH<sub>4</sub>Br(aq) <span class="ql-formula"
Q28: Complete the balancing of the following half-reaction,
Q29: When the equation, Al(s)+ NO<sub>3</sub><sup>-</sup>(aq)→ NO(g)+ Al<sup>3+</sup>(aq)is
Q31: When a copper metal reacts with aqueous
Q32: The CrO<sub>4</sub><sup>2-</sup> ion was involved in a
Q33: Balance the half-reaction, C<sub>2</sub>H<sub>6</sub>O <span
Q34: The ClO<sub>2</sub> molecule was involved in a
Q35: Balance the redox reaction below in acidic