Short Answer
A solution was made by taking 2.500 g of KMnO4 and dissolving it in enough water to make 1.000 liter of solution. This solution was used to titrate H2C2O4·2H2O, a very pure substance. In acidic media, the reaction is 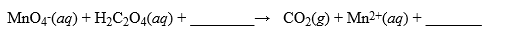 How many mL of this solution are required to titrate a 0.480 g sample of H2C2O4·2H2O? Hint: Remember to use moles as an intermediary when performing your stoichiometry calculations for the titration.
How many mL of this solution are required to titrate a 0.480 g sample of H2C2O4·2H2O? Hint: Remember to use moles as an intermediary when performing your stoichiometry calculations for the titration.
Correct Answer:

Verified
Correct Answer:
Verified
Q69: In the equation, CrO<sub>4</sub><sup>2-</sup>(aq)<sub> </sub>+ H<sub>2</sub>O(l)
Q70: Which statement is true concerning an oxidation-reduction
Q71: After balancing the following equation for
Q72: Fe<sup>2+</sup>(aq)reacts with MnO<sub>4</sub><sup>-</sup>(aq)ion in acidic solution
Q73: Balance the redox reaction below in acidic
Q75: When hydrocarbons or coal contain sulfur what
Q76: A partial activity series is: Pb <
Q77: What is the change in the
Q78: Complete the balancing of the following
Q79: In the equation, 2H<sup>+</sup> (aq)+ Zn(s)