Short Answer
It is proposed to measure the CO gas present in air samples by passing the gas through a solution of potassium permanganate. The reaction that occurs is, 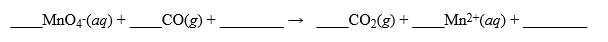 In a trial run, it required 102 liters of air containing CO (no other substances that would react with permanganate were present)to completely decolorize 250 mL of 0.0150 M MnO4-(aq)solution in a scrubber. If air weighs 1.29 g/liter, what is the percent by weight of CO in the air? Hint: Make sure your equation is balanced and pay attention to your units throughout the problem.
In a trial run, it required 102 liters of air containing CO (no other substances that would react with permanganate were present)to completely decolorize 250 mL of 0.0150 M MnO4-(aq)solution in a scrubber. If air weighs 1.29 g/liter, what is the percent by weight of CO in the air? Hint: Make sure your equation is balanced and pay attention to your units throughout the problem.
Correct Answer:

Verified
Correct Answer:
Verified
Q10: Ethanol, C<sub>2</sub>H<sub>5</sub>OH, is often found in gasoline.
Q11: Consider the redox equation, <br>2VO<sub>4</sub><sup>3-</sup>(aq)+ SO<sub>2</sub>(g)+
Q12: Magnesium metal reacts with aqueous sulfuric acid
Q13: The activity series of metals is
Q14: The reaction, Cl<sub>2</sub>(g)+ NaBr(aq) <span class="ql-formula"
Q16: What is the oxidation number of sulfur
Q17: In a chemical reaction, one of the
Q18: After balancing the following equation for
Q19: Fe<sup>2+</sup>(aq)reacts with MnO<sub>4</sub><sup>-</sup>(aq)ion in acidic solution
Q20: Balance the redox reaction below in basic