Short Answer
Each of the following solutions was treated with 1.00 M of H2SO4(aq). How many solutions will show a reaction? 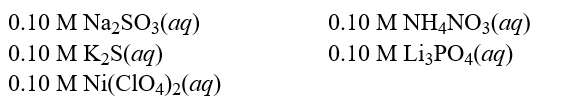 Hint: Consider spectator ions when determining if a reaction takes place.
Hint: Consider spectator ions when determining if a reaction takes place.
Correct Answer:

Verified
Correct Answer:
Verified
Q75: Which is NOT a strong acid?<br>A)HBr<br>B)HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub><br>C)HClO<sub>3</sub><br>D)HClO<sub>4</sub><br>E)HNO<sub>3</sub>
Q76: HNO<sub>2</sub> is a strong acid.
Q77: The equation for the reaction, AgNO<sub>3</sub>(aq)+ K<sub>2</sub>CrO<sub>4</sub>(aq)
Q78: In the reaction, KHS(aq)+ HCl(aq) <span
Q79: What is the formula for the oxoacid
Q81: An example of an acid-base neutralization is
Q82: How many grams of NH<sub>4</sub>NO<sub>3</sub> would be
Q83: Which statement below states a fact? Hint:
Q84: Which set below contains only weak acids?<br>A)HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>,
Q85: Addition of a sodium hydroxide solution to