Short Answer
Each of the following solutions was treated with 0.500 M of Na2CO3(aq). How many solutions will show a reaction? 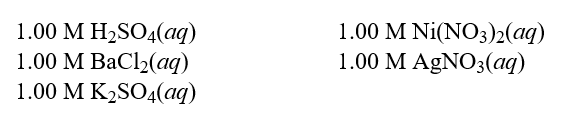 Hint: Consider spectator ions when determining if a reaction takes place.
Hint: Consider spectator ions when determining if a reaction takes place.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q184: Which statement is NOT correct? Hint: Remember
Q185: Each of the following solutions was treated
Q186: A solid laboratory sample contains two substances,
Q187: Limestone deposits are primarily composed of calcium
Q188: Most kidney stones are made up of
Q190: The symbol, H<sup>+</sup>(aq), is often used as
Q191: A solute is<br>A)a solid substance that does
Q192: A dynamic equilibrium is reached when hydrogen
Q193: What are the spectator ions for the
Q194: Write the equation for the ionization of