Multiple Choice
Select the examples in which the formulas do not correctly match the names of the compounds indicated. 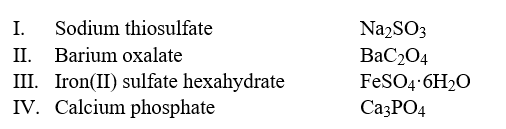 Hint: Write the correct name of each formula without looking at the names and then compare it to the choices.
Hint: Write the correct name of each formula without looking at the names and then compare it to the choices.
A) II only
B) II and III
C) I, II and IV
D) I and IV
E) II, III and IV
Correct Answer:

Verified
Correct Answer:
Verified
Q94: The formula for magnesium phosphide is Mg<sub>3</sub>P<sub>2</sub>.
Q95: Which compound exists as a diatomic molecule
Q96: Which compound is correctly represented as a
Q97: What is the formula of the compound
Q98: List the polyatomic ions, including the number
Q100: Which of these elements has the most
Q101: Which combination is used to represent molecular
Q102: The phosphide ion has 18 electrons and
Q103: The number of atoms in one formula
Q104: Oxygen reacts with a metal, which we