Multiple Choice
Which of the following five complexes are paramagnetic with 2 unpaired electrons? 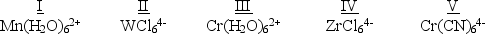 (If needed, use the following equation:Spectrochemical Series
(If needed, use the following equation:Spectrochemical Series
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) I and II
B) II, IV, and V
C) III
D) IV and V
E) I and III
Correct Answer:

Verified
Correct Answer:
Verified
Q28: What are reasons that metals are used
Q29: What is at the active site for
Q30: What is the layer above molten iron
Q31: The complex Fe(C<sub>2</sub>O<sub>4</sub>)<sub>3</sub><sup>3-</sup> has one unpaired electron.
Q32: What is the correct arrangement of Cr,
Q33: Which transition metal compounds are most likely
Q34: What is the oxidation state of rhodium
Q36: Which is the chemical formula for the
Q37: The transition metals with the electron configurations
Q38: Which of the following groups has primary