Short Answer
Butadiene, CH2=CH-CH=CH2 (line drawing below), forms polymers that are used in elastomers. Write the structure of polybutadiene (at least three units included) and explain why this polymer would be more elastic than polyethylene. 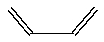
Correct Answer:

Verified
A section of a poly-butadiene molecule i...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q42: What is the linkage group and chemical
Q43: Proteins CANNOT be which of the following?<br>A)
Q44: Draw primary and secondary structures of DNA
Q45: Which of the following are likely to
Q46: Draw the line structure of the tripeptide
Q48: High density polyethylene<br>A) forms under conditions that
Q49: Draw a representative molecule containing three carbons
Q50: Nylon rope, a polyamide fibre product, has
Q51: What are the primary structures of the
Q52: What bonds CANNOT be easily broken in