Multiple Choice
What is the correct description, in line notation, for an electrochemical cell comprised of only Ag wire, AgNO3 electrolyte solution, and a salt bridge having G = -2 kJ?If needed, use the following equation: G° = -nFE°, E° 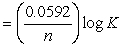 , E = E?
, E = E?
- 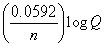 , moles e- =
, moles e- = 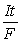 (If needed, refer to Table 17-1 in the text )
(If needed, refer to Table 17-1 in the text )
A) Ag(s) Ag+( aq, 1.00 M) Ag+(aq, 2.25 M) Ag(s)
B) Ag(s) Ag+( aq, 1.00 M) Ag+(aq, 1.00 M) Ag(s)
C) Ag(s) Ag+( aq, 2.25 M) Ag+(aq, 1.00 M) Ag(s)
D) Ag(s) Ag+( aq, 0.445 M) Ag+(aq, 1.00 M) Ag(s)
E) Ag(s) Ag+( aq, 1.00 M) , Ag+(aq, 2.25 M) Ag(s)
Correct Answer:

Verified
Correct Answer:
Verified
Q60: Ships, storage tanks, and other large metal
Q61: You have an abundant supply of NaCl
Q62: Calculate the standard free energy change
Q63: At an engine block rebuilding factory you
Q64: Which of the following combinations would provide
Q66: You determine that for proper protection of
Q67: How is aluminium protected from oxidation?(If needed,
Q68: Consider the redox reaction of permanganate
Q69: For the galvanic cell shown in the
Q70: Draw three molecular pictures illustrating direct electron