Short Answer
Consider the redox process:
Cd(OH)2 + Ni(OH)2 + 2 OH- NiO2 + Cd + H2OWrite the equation for the spontaneous process and determine the free energy change for the spontaneous process. 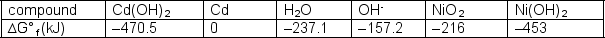
Correct Answer:

Verified
NiO2 + Cd + H2...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
NiO2 + Cd + H2...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q18: Consider an electrochemical cell of the type
Q19: For a brine electrolysis cell (see
Q20: Platinum metal is quite resistant to
Q21: Calculate the standard potential of voltaic cells
Q22: Use the half-reaction method to balance
Q24: An electrolytic cell driving the following
Q25: For the reaction given, which half
Q26: Assign oxidation numbers to all the elements
Q27: Calculate the standard potential of the
Q28: An electrochemical cell is made by