Short Answer
For the working galvanic cell shown at standard conditions, determine the balanced reaction and direction of electron flow through the wire. 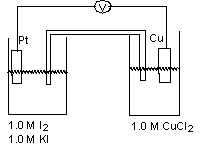 (If needed, refer to Table 17-1 in the text )
(If needed, refer to Table 17-1 in the text )
Correct Answer:

Verified
Electrons will flow from the c...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q39: Calculate the standard free energy change for
Q40: Consult a table of reduction potentials (Table
Q41: Calculate the standard free energy changes
Q42: What are the possible oxidation states of
Q43: Balance the following half reaction under
Q45: Consider the Daniell cell where the
Q46: Assign oxidation numbers to all the elements
Q47: For the reaction given below, which
Q48: Use oxidation numbers to show what is
Q49: Calculate the standard potential of the aluminium