Multiple Choice
Sodium carbonate, also called soda ash, is often analyzed by titration with strong acid. Initial concentrations of the carbonate ions are about 0.1 M. What indicator(s) would be suitable for detecting the stoichiometric point in this titration? (for H2CO3: pKa1 = 3.75 pKa2 = 10.33) 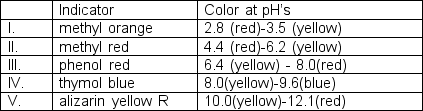
A) I only
B) I and II
C) III only
D) IV only
E) I and V
Correct Answer:

Verified
Correct Answer:
Verified
Q31: Which of the following solutions is a
Q32: What are the major species present when
Q33: A solution is prepared by adding 0.14
Q34: At 100°C, 2.1 x 10<sup>-2</sup> g of
Q35: A qualitative sketch of the titration curve
Q37: In which solution will copper nitrate (Cu(NO<sub>3</sub>)<sub>2</sub>)
Q38: At what pH will an 0.0010 M
Q39: In which of the following titrations will
Q40: Which of the following mixtures would make
Q41: Calculate the pH in the titration of