Short Answer
Calculate the pH in the titration of a 0.325 g sample of acetylsalicylic acid (see line drawing below) initially in 25.0 mL water with 0.102 M NaOH when (a) 8.84 mL and (b) 17.68 mL of titrant have been added. 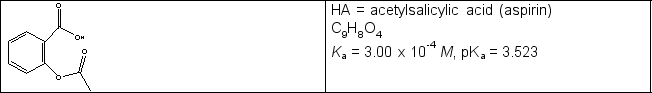
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q56: Two buffer solutions of the same pH
Q57: A 1.32 g sample of solid CuCl<sub>2</sub>•2H<sub>2</sub>O
Q58: What is the solubility product expression for
Q59: Write the formation constant expression for the
Q60: How many grams of AgNO<sub>3</sub> will dissolve
Q61: What is the pH upon adding 10
Q63: A solution is made by the addition
Q64: At what pH would a solution with
Q65: 1.1 mg of HgS (K<sub>sp</sub>1.6 x 10<sup>-54</sup>)
Q66: What mass of ammonium chloride (pK<sub>a</sub> (NH<sub>4</sub><sup>+</sup>)