Multiple Choice
Isomerization of hydrocarbons is important in the refining of petroleum. A simple example is that of butane, which has only two isomers: 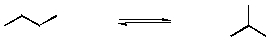
 Which of the following statements is most correct?
Which of the following statements is most correct?
A) At standard temperatures, n-butane is the preferred isomer.
B) At temperatures less than 450, n-butane is the preferred isomer.
C) At temperatures greater than 450ºK, n-butane is the preferred isomer.
D) At ambient temperatures, a sample of n-butane and isobutene which was at equilibrium would contain barely any butane.
E) A 1:1 mixture of butane and isobutane is at equilibrium at room temperature.
Correct Answer:

Verified
Correct Answer:
Verified
Q38: One mole of SF<sub>6</sub> (g) (S°= 291.82
Q39: At 45 <span class="ql-formula" data-value="\degree"><span class="katex"><span
Q40: The possible isomerization for ethanol to methyl
Q41: An important reagent in organic chemistry is
Q42: Predict the direction of change based on
Q44: A child plays with a deck of
Q45: What is the reaction quotient for
Q46: Which of the following selections has the
Q47: Chemical energy stored in glucose can
Q48: The process of storing energy for short