Multiple Choice
Use the following equation for questions
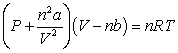
-The relative size of the Van der Waals constant, , correlates well with boiling point; that is, the larger is, the higher the boiling point. The reason for this correlation is
A) the constant is a measure of molecular size.
B) the boiling point is directly proportional to .
C) the constant varies with temperature.
D) the constant is a measure of intermolecular force strength.
E) the constant varies with vapour pressure.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: You have a solid that it characterized
Q2: Acetone CH<sub>3</sub>COCH<sub>3</sub><sub> </sub>boils at a significantly higher
Q3: Understand amorphous and crystalline solids at the
Q4: How much energy is required when 23
Q6: List at least two different physical properties
Q7: What is the main difference between an
Q8: Your solid is non-conductive and melts at
Q9: What are the differences in interparticle forces
Q10: Understand the effects of intermolecular forces on
Q11: Which of the following will have the