Multiple Choice
Below is the structure for zinc sulphide. If the zinc atoms (zinc) are contained within the unit cell and the sulphur atoms (clear) form a face-centered cubic structure, how many sulphur atoms must be contained within the unit cell to balance the charge? 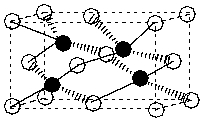
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer:

Verified
Correct Answer:
Verified
Q61: The figure shows the unit cell of
Q62: The face-centered cubic unit cell to the
Q63: Sketch a phase diagram for hydrazine locating
Q64: Arrange the following in order of increasing
Q65: Na<sup>+ </sup>has an ionic radius of 116
Q66: The leaves of the lotus plant are
Q67: Which type of solid is the most
Q68: Draw molecular pictures that illustrate and explain
Q70: Arrange the following in order of increasing
Q71: Describe how trees are able to transport