Multiple Choice
Consider the van der Waals equation: 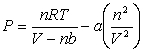
A) the van der Waals "a" term is proportional to molecular size and "b" is proportional to the magnitude of intermolecular forces.
B) the van der Waals "b" term is proportional to molecular size and "a" is proportional to the magnitude of intermolecular forces.
C) large molecules tend to have smaller values for both a and b.
D) the van der Waals equation describes successfully describes the behaviour of non-ideal gases.
Correct Answer:

Verified
Correct Answer:
Verified
Q7: Consider a sample of gas in a
Q8: If the relative humidity at 28°C is
Q9: The level of mercury on the sample
Q10: A sample of gas is cooled to
Q11: Consider the van der Waals "a" coefficient
Q13: Which will occupy a larger volume, 10
Q14: Cooling the gas in a container lowers
Q15: If the compressibility factor, pV/nRT is 1
Q16: Methylamine, whose line structure is shown below,
Q17: If 760 Torr is equivalent to 1