Short Answer
Use the following information for Questions
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)
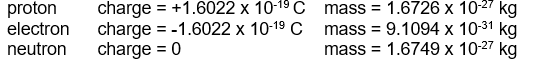
-You are asked to administer 4.9 g of an injectable solution which contains 0.23 % of a lifesaving drug by mass. The syringe has graduations of 0.1 mL. If the density of the solution is 1.13 g/mL, how many mL should you inject?
Correct Answer:

Verified
Correct Answer:
Verified
Q35: What are two microscopic properties of a
Q36: Use the following information for Questions<br>3.785 L
Q37: Adipic acid, whose line structure is shown
Q38: When 500 ml of 1.75 M
Q39: Use the following information for Questions<br>3.785 L
Q41: Use the following information for Questions .<br>1
Q42: Calculate yields of chemical reactions.
Q43: What volume of 1.0 M NaCl solution
Q44: A way of generating dry O<sub>2</sub> in
Q45: Solve limiting-reagent-type problems.