True/False
The equilibrium constant expression for the reaction, 2 HgO(s)? 2 Hg(l)+ O2(g)is K = .
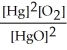
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q70: Consider the following system at equilibrium: N<sub>2</sub>
Q71: When the pH of a solution of
Q72: As the pH value increases, [H<sub>3</sub>O<sup>+</sup>] _.
Q73: In a basic solution, pH is _
Q74: The strength of an acid increases as
Q75: Calculate the pH of 0.00756 M HNO<sub>3</sub>.<br>A)11.879<br>B)7)091<br>C)2)121<br>D)12.947
Q76: Which of the following represents the zwitterions
Q77: A large value of K means that
Q78: Which of the following is not a
Q80: A Brønsted-Lowry acid is defined as a