Multiple Choice
Which of the following alkanes has the highest boiling point?
A) CH3CH2CH2CH2CH2CH3
B) 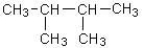
C) 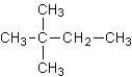
D) 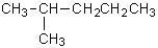
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q67: In sublimation a solid is converted into
Q68: Induced dipole and dispersion forces describe the
Q69: Lipids that have the fused ring structure
Q70: Straight chain alkanes have lower boiling points
Q71: London dispersion forces attractions between molecules depends
Q73: Which one of these molecules can act
Q74: Which of the following statements is not
Q75: _ attractions are the only ones that
Q76: Sugar dissolves in water because of the
Q77: Which of these alkanes has the lowest