Multiple Choice
Which compound would have the highest boiling point?
A) CH3-O-CH2CH2CH3
B) CH3CH2-O-CH2CH3
C) 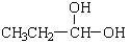
D) CH3CH2CH2CH2-OH
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q58: What is the difference between the terms
Q59: Let's compare two compounds of similar molar
Q60: When NaCl dissolves in water, the force
Q61: Rank the following compounds in order of
Q62: Which of the following is most likely
Q64: Dipole-dipole attractions exist between molecules that have
Q65: The transition from the gas phase directly
Q66: The polar heads of soap molecules are:<br>A)hydrophilic
Q67: In sublimation a solid is converted into
Q68: Induced dipole and dispersion forces describe the