Short Answer
When temperature is held constant, the pressure P and volume V of a quantity of gas are inversely proportional (Boyle's Law). The following figure shows this relationship for a particular gas. Find a formula for V in terms of P and use it to find V when P is 12. Round to 2 decimal places.
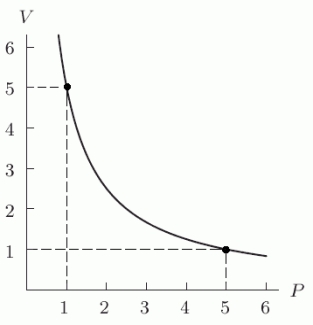
Correct Answer:

Verified
Correct Answer:
Verified
Q44: The formula for the polynomial function of
Q45: A 16 kg sample of a certain
Q46: Find the equation of the horizontal line
Q47: Which of the following statements are true
Q48: The formula for the power function shown
Q50: What are the <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10073/.jpg" alt="What
Q51: The number of yearly childbirths for a
Q52: Find the formula for a third degree
Q53: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10073/.jpg" alt=" " class="answers-bank-image d-block" rel="preload"
Q54: The sum of two odd functions is