Multiple Choice
The most energetic electron in any atom at the beginning of a period of the periodic table is in:
A) an 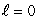 state
state
B) an 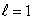 state
state
C) an 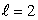 state
state
D) an n = 0 state with unspecified angular momentum
E) an n = 1 state with unspecified angular momentum
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: An electron in an atom is
Q14: The group of atoms at the beginning
Q15: To observe the Zeeman effect one uses:<br>A)
Q16: A magnetic dipole <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10137/.jpg" alt="A magnetic
Q17: A laser beam can be sharply focused
Q18: A laser must be pumped to achieve:<br>A)
Q19: No state in an atom can be
Q21: An electron in an atom is
Q22: The number of possible values of the
Q23: The Pauli exclusion principle is obeyed by:<br>A)