Multiple Choice
Solve the problem.
-According to the Gas Kinetic Theory, the average speed of a gas particle is given by 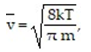 where
where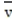 is the speed in m/s, k is the constant 1.38 × 10-23 , T is the temperature of the gas in Kelvin, and m is the mass of the gas particle in kg. What is the average speed of an oxygen molecule with a mass of
is the speed in m/s, k is the constant 1.38 × 10-23 , T is the temperature of the gas in Kelvin, and m is the mass of the gas particle in kg. What is the average speed of an oxygen molecule with a mass of
5.314 X 10-26 KG at a temperature of 500 K?
A) 1020 m/s
B) 575 m/s
C) 330,700 m/s
D) 174 m/s
Correct Answer:

Verified
Correct Answer:
Verified
Q191: Solve the problem.<br>-Find the point on the
Q192: Find the limit.<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9662/.jpg" alt="Find the limit.
Q193: Write a chain rule formula for the
Q194: Find all the second order partial derivatives
Q195: Find the absolute maxima and minima of
Q197: Write a chain rule formula for the
Q198: Find all local extreme values of the
Q199: Find the domain and range and describe
Q200: Find the extreme values of the function
Q201: Compute the gradient of the function at