Multiple Choice
Solve the problem.
-The Van der Waals equation provides an approximate model for the behavior of real gases. The equation is P(V, T) = 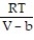 -
- 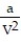 , where P is pressure, V is volume, T is Kelvin temperature, and a,b , and R are constants. Find the partial derivative of the function with respect to each variable.
, where P is pressure, V is volume, T is Kelvin temperature, and a,b , and R are constants. Find the partial derivative of the function with respect to each variable.
A) 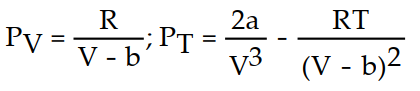
B) 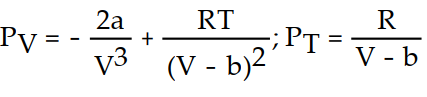
C) 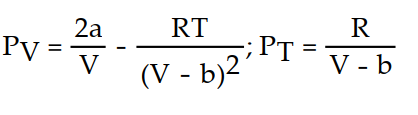
D) 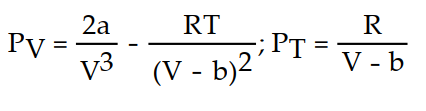
Correct Answer:

Verified
Correct Answer:
Verified
Q119: Find the limit.<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9662/.jpg" alt="Find the limit.
Q120: Write a chain rule formula for the
Q121: Use implicit differentiation to find the specified
Q122: Solve the problem.<br>-Write an equation for the
Q123: Solve the problem.<br>-Find the equation for the
Q125: Find the domain and range and
Q126: Solve the problem.<br>-Find the derivative of the
Q127: Write a chain rule formula for the
Q128: Compute the gradient of the function at
Q129: Find the domain and range and describe