Essay
Predict the products of the following acid-base reaction.
NH4Cl(aq) + NaSH(aq) 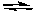
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q85: Which of the following won't produce an
Q86: For H<sub>2</sub>CO<sub>3</sub>, K<sub>a2</sub> = 4.7 x 10<sup>-11</sup>.
Q87: Which of the following compounds cannot be
Q88: The base-ionization constants for three bases, A(OH),
Q89: HA is a weak monoprotic acid with
Q91: Assume a solution is prepared by
Q92: If equimolar amounts of NH<sub>3</sub> and NH<sub>4</sub>Cl
Q93: Using a table of K<sub>a</sub>'s, determine which
Q94: Use the following acid-dissociation equilibrium constants for
Q95: Which of the following would form the