Multiple Choice
NaHCO3 can be used to neutralize strong bases, such as NaOH. What conclusion can be drawn from the fact that the following acid-base reaction proceeds to the right as written?
HCO3-(aq) + OH-(aq) 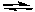 CO32-(aq) + H2O(l)
CO32-(aq) + H2O(l)
A) HCO3- is a stronger acid than H2O.
B) HCO3- is a stronger base than CO32-.
C) HCO3- is a stronger base than OH-.
D) CO32- is a stronger base than OH-.
E) H2O is a stronger acid than HCO3-.
Correct Answer:

Verified
Correct Answer:
Verified
Q43: Sulfurous acid (H<sub>2</sub>SO<sub>3</sub>) is an acid and
Q44: When NH<sub>4</sub>Cl is dissolved in water the
Q45: A solution is known to have a
Q46: Which of the following compounds is not
Q47: Which of the following represents a conjugate
Q49: Which equation best describes a 0.10
Q50: Which of the following compounds aren't acids
Q51: Use the relationship between K<sub>a1</sub> and
Q52: For a particular weak acid, HA, in
Q53: Which of the following equations can be