Multiple Choice
What is the pH of a solution that is simultaneously 0.10 M in both NH3 and the NH4+ ion?
(NH4+: Ka = 5.6 x 10-10)
NH3(aq) + H2O(l) 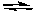 NH4+(aq) + OH-(aq)
NH4+(aq) + OH-(aq)
A) 4.75
B) 7.00
C) 9.25
D) The pH of this solution would be impossible to predict.
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q79: Which of the following structural factors is
Q80: Arrange the following bases in order of
Q81: Which one of the following mixtures would
Q82: Succinic acid (HO<sub>2</sub>CCH<sub>2</sub>CH<sub>2</sub>CO<sub>2</sub>H) is an intermediate
Q83: Use the following information to answer <br>H<sub>2</sub>Se:
Q85: Which of the following won't produce an
Q86: For H<sub>2</sub>CO<sub>3</sub>, K<sub>a2</sub> = 4.7 x 10<sup>-11</sup>.
Q87: Which of the following compounds cannot be
Q88: The base-ionization constants for three bases, A(OH),
Q89: HA is a weak monoprotic acid with