Multiple Choice
What is the pH of a 0.10 M succinic acid, H2Sc, solution if we assume that H2Sc is a weak acid that undergoes stepwise dissociation?
H2Sc(aq) + H2O(l) 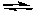 H3O+(aq) + HSc-(aq) Ka1 = 6.9 x 10-5
H3O+(aq) + HSc-(aq) Ka1 = 6.9 x 10-5
HSc-(aq) + H2O(l) 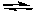 H3O+(aq) + Sc2-(aq) Ka2 = 2.5 x 10-6
H3O+(aq) + Sc2-(aq) Ka2 = 2.5 x 10-6
A) 2.1
B) 2.6
C) 3.3
D) 4.2
E) 5.6
Correct Answer:

Verified
Correct Answer:
Verified
Q1: What is the [H<sub>3</sub>O<sup>+</sup>] concentration of a
Q2: The dihydrogen phosphate ion, H<sub>2</sub>PO<sub>4</sub> <sup>-</sup> ,
Q3: A strong acid is added to water.
Q4: What is the OH<sup>-</sup> ion concentration in
Q5: What is the K<sub>b</sub> for methylamine (CH<sub>3</sub>NH<sub>2</sub>)
Q7: Which of the following is the conjugate
Q8: Which of the following statements should be
Q9: A solution is prepared by dissolving 1.0
Q10: Using data from the previous problem, what
Q11: Which of the following statements is true?<br>A)