Multiple Choice
The following questions often assume that a truncated table of standard-state thermodynamic data is attached to the exam.
Specific Heat and Heat Capacity
-How much heat in joules is produced by mixing 50.0 mL of 1.0 M HBr at 25.6°C with 50.0 mL of 1.0 M KOH at 25.6°C if this reaction produces 100 mL of a solution with a temperature of 32.3°C? (Assume the heat capacity of water is 4.18 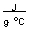 and the density of these solutions is 1.00
and the density of these solutions is 1.00 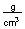 )
)
A) 6.7 J
B) 670 J
C) 1400 J
D) 2800 J
E) 5600 J
Correct Answer:

Verified
Correct Answer:
Verified
Q26: The following questions often assume that a
Q27: Consider the following data for heats of
Q28: The following questions often assume that a
Q29: (Note that some of these
Q30: Use your understanding of the bonding
Q32: Use the following standard enthalpies of
Q33: Which of the following reactions is
Q34: The enthalpy of atom combination for CCl<sub>4</sub>(g)
Q35: Arrange the following in order of increasing
Q36: How much heat in kilojoules is