Essay
The following reaction occurs when sucrose (cane sugar) is metabolized by the body.
C12H22O11(s) + 12 O2(g) 12 CO2(g) + 11 H2O(l)
Assume that Hrxn° for this reaction is -5645 kJ/molrxn. What is the value of Hac° for sucrose?
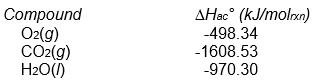
Correct Answer:

Verified
-18,350 kJ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q49: (Note that some of these
Q50: Hydrogen peroxide is a good oxidizing
Q51: The change in enthalpy, <span
Q52: (Note that some of these
Q53: Write a balanced equation for the
Q55: Which of the following reactions is
Q56: At what temperature are standard-state enthalpy of
Q57: The following questions often assume that a
Q58: Arrange the following in order of increasing
Q59: Which of the following reactions is