Multiple Choice
The bond-type triangle can be used for
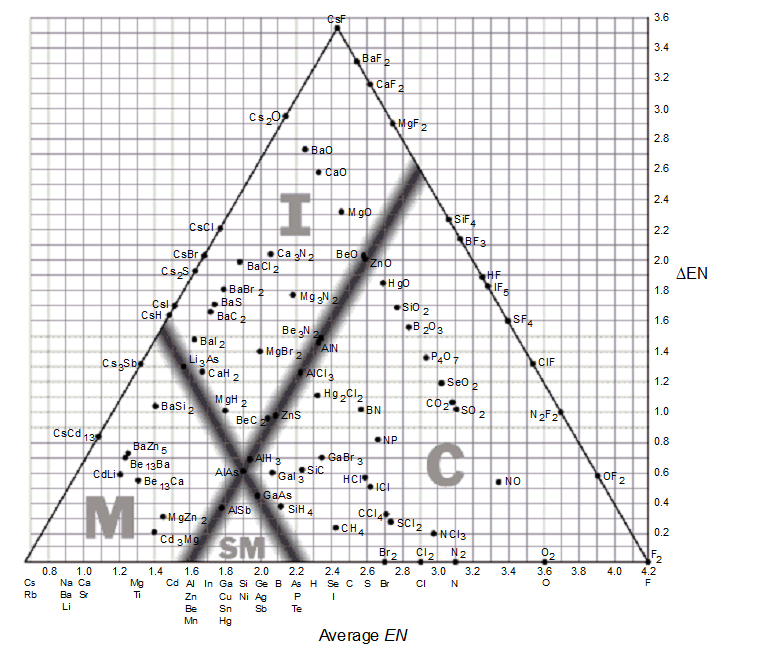
-Based on the bond-type triangle, which of these covalent compounds has the greatest ionic character?
A) NCl3
B) SCl2
C) ICl
D) HCl
E) SiC
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q38: Which of the following is the most
Q39: The two most common ions of copper
Q40: The bond-type triangle can be used for
Q41: What is the formula of the neutral
Q42: The name for Li<sub>3</sub>N is:<br>A) lithium nitride<br>B)
Q44: What would be the product of the
Q45: In the following oxidation-reduction reaction, which
Q46: Honda and Toyota both sell hybrid cars
Q47: Give the chemical formula for barium chromate.<br>A)
Q48: If the formula for aluminum oxide is