Multiple Choice
The bond-type triangle can be used for
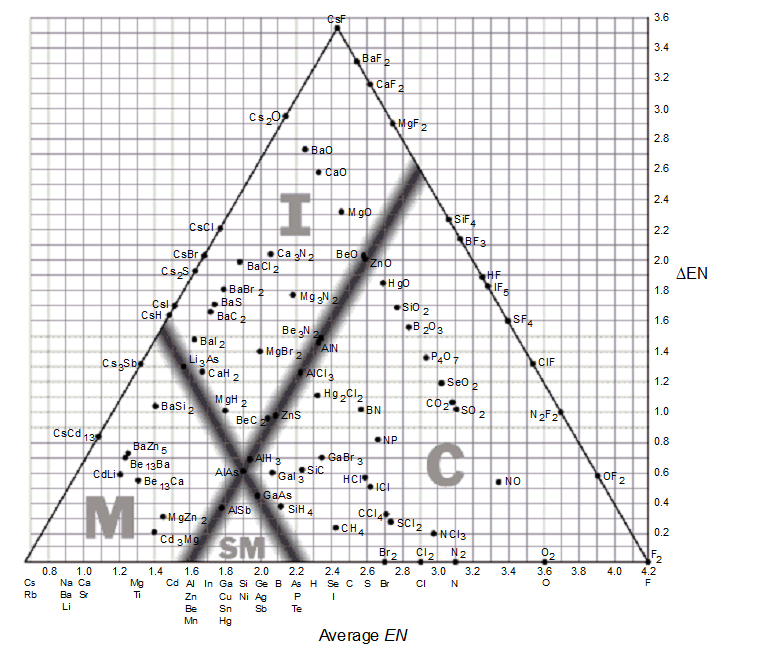
-According to the supplied bond-type triangle, which of the following compounds is most likely to conduct electricity as a solid?
A) SO2
B) MgBr2
C) CdLi
D) NCl3
E) N2F2
Correct Answer:

Verified
Correct Answer:
Verified
Q68: What is the most likely charge for
Q69: Which isn't an oxidation-reduction reaction?<br>A) <img
Q70: Give the chemical formula for ammonium bromide.<br>A)
Q71: In Chapter 4 we were able to
Q72: Use the positions of gallium and oxygen
Q74: If the difference in electronegativity between two
Q75: A main-group <span class="ql-formula" data-value="\underline{\text{
Q76: All but one of the following species
Q77: Give the name for the compound Fe<sub>2</sub>O<sub>3</sub>.<br>A)
Q78: Determine the oxidation number of chlorine in