Multiple Choice
Which is a legitimate set of n, l, ml and ms quantum numbers?
A) 0,0,0, 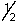
B) 8,4,-3,- 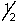
C) 3,3,2,+ 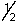
D) 2,1,-2,- 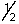
E) 5,3,3,-1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q31: Give the orbital diagram (using parentheses and
Q32: For each pair of atoms, which has
Q33: What is the lowest energy electronic configuration
Q34: What is the orbital designation for the
Q35: Predict which atom is most likely to
Q37: Which element is represented by the following
Q38: Which of the following atoms would have
Q39: A possible set of quantum numbers for
Q40: Which of the following quantum numbers is
Q41: Predict which atom is most likely to