Multiple Choice
Which of the following sets of n, l, ml and ms quantum numbers isn't allowed?
A) 1,1,0,+ 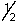
B) 2,0,0,+ 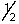
C) 3,2,1,- 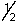
D) 4,1,-1,+ 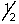
E) 5,3,2,- 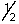
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: Photochemistry is when light causes a chemical
Q21: The outermost or highest energy electron in
Q22: Which of the following atoms or ions
Q23: Which atom is represented by a shell
Q24: What is the value of x in
Q26: The Pauli exclusion principle states that:<br>A) No
Q27: Which of the following subshells is filled
Q28: The first ionization energy of the first
Q29: The line structure of the emission spectrum
Q30: If X-rays have a shorter wavelength than