Multiple Choice
Which of the following describes a possible set of quantum numbers for the last electron added to form an aluminum atom when atomic orbitals are filled?
A) 1, 0, 0, + 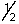
B) 2, 0, 0, + 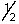
C) 2, l, 1, + 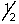
D) 3, 0, 0, + 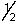
E) 3, 1, 1, + 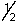
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q84: Given the information below, what is the
Q85: There is a fundamental difference between
Q86: What is the energy of the light
Q87: A cheap spectrophotometer can be made using
Q88: Predict the order of decreasing first ionization
Q90: In a 23.490 kilogauss magnetic field, <sup>13</sup>C
Q91: Yellow light has a frequency of 5.2
Q92: Which of the following atoms or ions
Q93: Which set of n, l, m<sub>l</sub> and
Q94: If a laser operated on the n