Multiple Choice
A cyclic process is carried out on an ideal gas such that it returns to its initial state at the end of a cycle.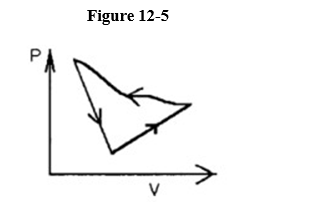
If the process was carried out in a counter-clockwise sense around the enclosed area, as shown on the p-V diagram in Figure 12-5, then that area represents
A) the work done on the ideal gas.
B) the heat added to the ideal gas.
C) the work done by the ideal gas.
D) the heat that flows from the ideal gas.
Correct Answer:

Verified
Correct Answer:
Verified
Q50: The most efficient engine possible is the<br>A)
Q51: If the theoretical efficiency of a Carnot
Q52: 250. J of work is done in
Q53: A gas is allowed to expand at
Q54: A substance is taken through the illustrated
Q56: A gas is taken through the cycle
Q57: When the first law of thermodynamics,
Q58: Being the ideal engine, the maximum COP
Q59: Consider two cylinders of gas identical in
Q60: Consider a Carnot refrigerator which is operated